Article Summary:
Carbon is a versatile element with diverse conductive properties depending on its structure and form. This article explores the fundamental question: “Is carbon conductive?” It explains how different forms of carbon; graphite, diamond, amorphous carbon, and carbon black vary in conductivity.
We then delve into carbon black as a conductive material and discuss its properties, uses, and advantages. Finally, we compare carbon black to Austin Black 325, a natural black filler with distinct characteristics, focusing on their conductivity and applications in various industries.
Introduction
Carbon is an essential element in various industrial applications, often chosen for its unique ability to conduct electricity.
However, not all forms of carbon exhibit the same conductivity. While graphite is known for its high conductivity, diamond, another form of carbon, is an excellent insulator.
The question of whether carbon is conductive is complex, and understanding its answer requires exploring the structure, properties, and forms of carbon.
In this article, we’ll break down carbon’s conductivity, examine the role of carbon black as a conductive material, and compare it to Austin Black 325, offering insights into their practical applications.
Forms of Carbon and Their Conductivity
The conductivity of carbon is not a fixed trait but varies depending on its atomic structure and bonding. Below is a table that summarizes the different forms of carbon and their conductive properties:
| Form of Carbon | Structure | Conductivity | Applications |
| Graphite | Layers of hexagonal carbon lattices with free-moving electrons. | High conductivity due to delocalized electrons. | Batteries, electrodes, lubricants. |
| Diamond | Tightly bonded tetrahedral lattice. | Non-conductive (excellent insulator). | Cutting tools, thermal conductors. |
| Amorphous Carbon | Irregular structure with mixed bonding types. | Moderate to low conductivity. | Pigments, electrodes, activated carbon. |
| Carbon Black | Nearly pure carbon with high surface area and small particles. | Good conductivity when dispersed in materials. | Rubber reinforcement, conductive coatings. |
- Key Takeaway: Graphite’s layered structure makes it highly conductive, while diamond’s rigid structure blocks electron flow. Carbon black’s conductivity falls between these extremes and depends on its production and properties.
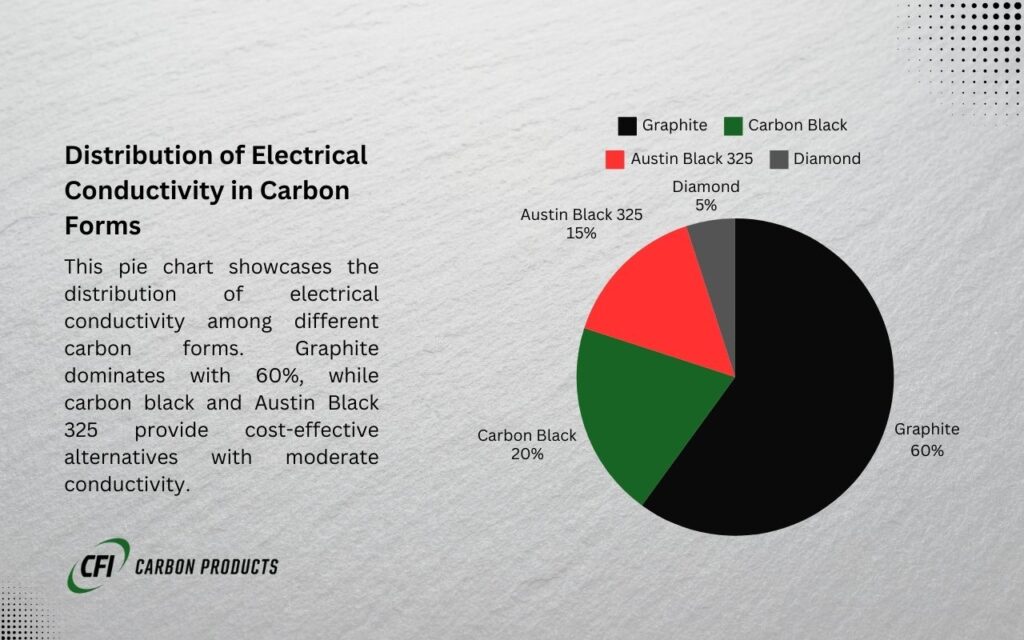
Carbon Black as a Conductive Material
Carbon black is an industrially significant form of carbon with unique properties that make it highly versatile.
It is manufactured by the incomplete combustion of hydrocarbons, resulting in fine particles with a high surface area.
Properties of Carbon Black:
| Property | Description |
| Particle Size | Very small particles (10–100 nm) that enhance conductivity and dispersion. |
| Surface Area | High surface area increases electrical and thermal conductivity. |
| Structure | Aggregated particles that form chain-like structures for better reinforcement. |
| Purity | Nearly pure elemental carbon, free of impurities that could affect performance. |
Applications of Carbon Black
- Rubber and Tires: Reinforces rubber and provides electrical conductivity in anti-static tires.
- Plastics and Polymers: Enhances UV resistance and conductivity in plastic parts.
- Coatings and Paints: Provides tinting strength and conductivity for anti-static and protective coatings.
- Batteries: Acts as a conductive agent in electrodes for lithium-ion batteries.
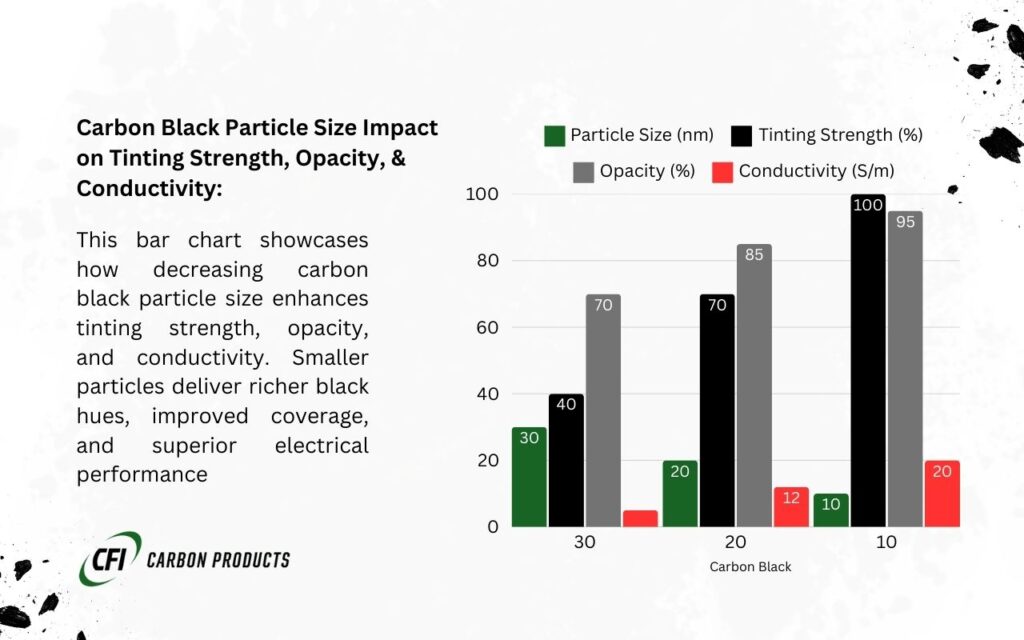
Why Carbon Black is Conductive:
Its small particle size and high surface area allow electrons to move efficiently between particles, improving the material’s overall conductivity.
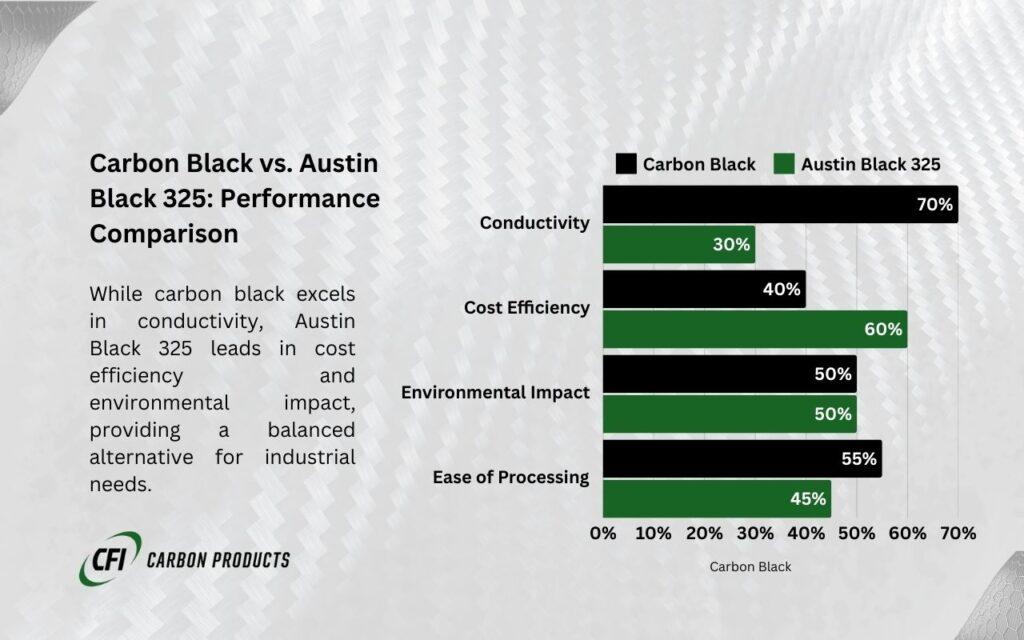
Comparing Carbon Black to Austin Black 325
While carbon black is engineered for specific conductivity and reinforcement properties, Austin Black 325 is a naturally sourced black filler made from ground coal. It offers distinct advantages in cost-effectiveness and pigmentation but has lower conductivity compared to carbon black.
Key Comparisons
| Feature | Carbon Black | Austin Black 325 |
| Origin | Produced from the incomplete combustion of hydrocarbons. | Natural filler derived from coal. |
| Conductivity | High conductivity due to small particle size and high surface area. | Low conductivity; not designed for electrical applications. |
| Particle Size | 10–100 nm for high dispersion and performance. | Larger particle size (~10 µm). |
| Applications | Conductive coatings, batteries, and reinforced rubber. | Cost-efficient filler for pigmentation. |
When to Use Each
- Carbon Black: Ideal for applications requiring electrical conductivity, UV resistance, and reinforcement (e.g., tires, batteries).
- Austin Black 325: Suitable for cost-sensitive applications where conductivity isn’t a priority but pigmentation and filler properties are needed (e.g., asphalt, low-cost rubber products).
Practical Applications and Considerations
To choose between carbon black and Austin Black 325, manufacturers must consider the following factors:
- Electrical Requirements: Use carbon black for conductive needs and Austin Black 325 for non-conductive applications.
- Cost Efficiency: Austin Black 325 is a more economical option for large-scale use.
- Environmental Impact: Both materials have minimal environmental impact when used responsibly, but carbon black production involves combustion processes.
Conclusion
Carbon’s conductivity depends on its structure, with graphite and carbon black exhibiting high conductivity and other forms, like diamond, being insulating.
Carbon black’s engineered properties make it indispensable for industries requiring conductive materials, while Austin Black 325 offers a cost-effective alternative for non-conductive applications.When selecting between the two, it’s crucial to balance performance, cost, and application-specific needs. Consulting experts or suppliers ensures the best material choice for your requirements.


